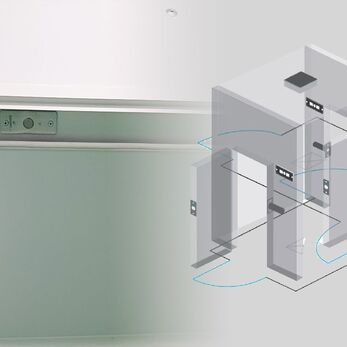
Interlock is an interlocking system that prevents multiple doors from being opened simultaneously in an airlock, maintains pressure, and prevents cross-contamination. So why is interlock a mandatory device in GMP airlocks? This article will analyze the role, principles, and benefits of this device in cleanroom control.
- 1. What is Interlock? Operating mechanism in cleanrooms
- 2. The role of interlock in GMP airlock systems
- 3. GMP scenarios where interlock is mandatory
- 4. Consequences of not using a compliant interlock system
- 5. Key criteria for choosing the right interlock for GMP facilities
- 6. Interlock system recommendations by industry
- 7. Frequently Asked Questions about interlocks in GMP cleanrooms
- 8. Contact us for interlock consultation tailored to your GMP facility
1. What is Interlock? Operating mechanism in cleanrooms
Interlock is a door interlocking system between two or more doors, ensuring that only one door can be opened at a time, while the others remain locked. It is a critical solution in cleanroom design to prevent uncontrolled airflow and cross-contamination between areas with different cleanliness levels.
This system is typically installed at transition points such as airlocks, where personnel or materials pass between clean and non-clean environments, helping to maintain stable cleanliness in the production area.
Operating principle of interlock
When one door in the interlock system is opened:
- The remaining doors are automatically locked until the open door is fully closed.
- Some advanced systems integrate sensors to detect door status and alert if doors are opened simultaneously or not fully closed.

This mechanism prevents simultaneous door openings, avoiding unwanted air turbulence that may carry dust, microbes, or contaminants into the clean zone.
Types of interlock in cleanrooms
|
Interlock Type |
Control Mechanism |
Main Advantage |
Limitation |
Suitable Application |
|
Mechanical interlock |
Pure mechanical (rods, levers) |
Low cost, no electricity needed |
Less flexible, hard to expand |
Small facilities with basic requirements |
|
Electrical interlock |
Relays, basic switches |
Stable operation, easy to install |
Limited smart functions |
Medium-sized facilities, simple zoning |
|
Electronic interlock |
Electronic circuits, microcontrollers |
Integrates alerts and sensors |
Higher cost |
GMP facilities with strict control needs |
|
Smart interlock |
PLC or BMS integration |
Flexible control, remote monitoring |
Requires technical configuration |
Large factories with complex systems |
2. The role of interlock in GMP airlock systems
Enforcing clean zone hierarchy - preventing cross-contamination
In GMP-compliant (Good Manufacturing Practice) facilities, maintaining cleanliness segregation is mandatory to minimize contamination by particles, dust, or microbes. Interlock systems act as “invisible gatekeepers” by ensuring:
- Personnel cannot open both doors connecting the hallway and production area simultaneously.
- Materials are transferred through pass boxes or airlocks following strict protocols, avoiding cross-contamination between clean and unclean zones.
Thus, interlocks help manage unidirectional movement, keeping cleanliness levels isolated and stable—especially crucial in weighing rooms, filling zones, and packaging areas.
Controlling pressure differentials between zones
One of GMP’s core requirements is maintaining a stable pressure differential (usually ≥10 Pa) between rooms of different cleanliness classes. If both doors in an airlock are open at once, clean air may escape or reverse, leading to:
- Contaminants from hallways entering cleanrooms
- Uncontrolled airflow → affecting production outcomes
Interlock systems ensure sequential door operation, preventing “short-circuit” airflows and preserving pressure zoning integrity within HVAC systems.

Preserving production zone integrity under GMP
Especially in the pharmaceutical industry—where every gram of material impacts patient safety—interlocks are essential to preserve production integrity.
Properly implemented interlocks help:
- Prevent human error (e.g., opening both doors simultaneously)
- Enhance automation and monitoring in contamination control
- Support compliance with audit requirements during GMP inspections
See more: Latest price list of Interlock used in Cosmetic factory
3. GMP scenarios where interlock is mandatory
Interlock systems are not just a technical option, but a mandatory requirement under GMP standards, especially in pharmaceutical, cosmetics, and nutraceutical plants certified under EU-GMP, WHO-GMP, and PIC/S. Below are typical cases where interlocks must be integrated from the design stage.
Airlocks between production areas and common corridors
These are transition points between clean and uncontrolled environments—the highest risk zones for contamination. Without interlocks, both doors may open simultaneously, causing positive pressure loss and potential backflow of unclean air.
GMP requires interlocks in airlocks that separate production zones and hallways, to:
- Prevent simultaneous opening of inner and outer doors
- Ensure airflow always moves from cleaner to less clean areas
Changing rooms - packaging areas - weighing rooms
These are critical transition points for both personnel and materials. Notably:
- Changing rooms: Interlocks regulate the step-by-step gowning procedure, preventing door mistakes and maintaining unidirectional changeover.
- Weighing rooms: High risk of dust dispersion—simultaneous door opening may spread raw material powder into adjacent areas.
- Packaging zones: Without interlocks, paper dust or packaging debris may backflow into production areas, causing cross-contamination.
Requirements from EU-GMP and WHO-GMP
In guidelines such as EU-GMP Annex 1 or WHO TRS 961, interlock systems are highlighted as part of the “pressure cascade” strategy and unidirectional flow design.
Key requirements include:
- All airlocks must be designed to allow only one door to open at a time
- Interlocks may be mechanical or electronic but must be stable and verifiable
- In emergencies (fire, power outage), the system must have a fail-safe opening mechanism
4. Consequences of not using a compliant interlock system
Failing to install or correctly operate interlock systems in areas such as airlocks, changing rooms, or weighing rooms can result in more than just operational inconvenience. In strictly controlled environments like pharmaceutical and cosmetic production, such negligence can lead to serious consequences affecting the entire facility.
Loss of airflow control - Increased risk of cross-contamination
Interlocks maintain door sequencing, ensuring cleanroom areas retain ideal pressure differentials. If doors are opened simultaneously:
- Clean air may escape → allowing dust and microorganisms from dirty zones to enter
- Airflow can reverse, disrupting contamination control
This results in cross-contamination between products or raw materials, one of the most critical GMP violations—possibly leading to product recalls or production suspension.
Failure in GMP audits - Risk of warnings or suspension
During inspections or GMP validation, auditors will evaluate:
- Door control mechanisms in airlock zones
- Whether SOPs include interlock procedures
- If the system has fail-safe operation during power outage or fire
If interlocks are missing, faulty, or misused, the facility may:
- Receive warnings from regulatory agencies (e.g., CAPA, OOS)
- Be required to retrofit entire airlock systems
- Face temporary suspension until compliance is restored

Difficulty proving compliance during validation/approval
When applying for GMP certification or product registration, factory documentation must demonstrate:
- Effective control of personnel/material flow and pressure differentials
- All airlocks follow unidirectional movement principles
Without interlocks, flow diagrams on P&ID or cleanroom layouts may lack credibility. This can delay audits, require repeated document submissions, and prolong timelines for operation or product launch.
See more: Prepping for Success: Essential Considerations Before Cleanroom Door Interlock Installation
5. Key criteria for choosing the right interlock for GMP facilities
Not all interlock systems are suitable for GMP manufacturing environments. Choosing the right type ensures cleanroom stability, efficient operation, and smooth GMP validation. Below are three critical criteria for selecting interlocks in pharmaceutical, cosmetics, or nutraceutical plants.
1. Compatibility with HVAC and BMS systems
In modern GMP factories, interlocks should not function independently. They must integrate with HVAC (Heating, Ventilation, and Air Conditioning) and BMS (Building Management Systems) to:
- Automatically lock doors if pressure is not within specifications
- Transmit door open/close signals to monitor pressure control
- Log door activity in central monitoring systems
Tip: Prioritize interlocks using Modbus or industrial-grade relays for easier integration and system synchronization.
2. Safety features: fail-safe and alarm notifications
A GMP-compliant interlock must include safety mechanisms to protect personnel and maintain process integrity:
- Fail-safe operation: Doors automatically switch to a predefined safe state (open or locked) in case of power loss
- Alarm notifications: Audio/visual alerts when two doors are opened simultaneously or a door is not fully closed
- Emergency override: Allows staff to manually open doors during emergencies (fire, gas leaks, etc.)
3. Scalability, connectivity, and easy maintenance
GMP plants often have multiple airlocks and zones with potential for expansion. Your interlock system should offer:
- Control for multiple doors (2 to 6), with flexible grouping
- Easy software upgrades or hardware additions (fire alarms, fingerprint readers, HMI screens)
- Simple maintenance with replaceable components, clear instructions, and strong technical support
Suggestion: For large-scale facilities, use PLC- or HMI-based centralized control interlocks that can monitor the status of all doors from one control panel.
See more: What is Cleanroom Airlock? A Crucial Safeguard in GMP Facilities
6. Interlock system recommendations by industry
Each GMP-regulated industry has unique environmental and operational requirements. Therefore, interlock systems should be tailored to meet industry-specific standards while balancing cost-efficiency and usability.
Pharmaceutical industry: strict control - integrated alarms
In pharmaceutical manufacturing, airlocks often separate zones with multiple cleanliness levels. Interlocks must:
- Manage 3-4+ doors with flexible opening sequences
- Include audio/visual alarms for incorrect door usage
- Integrate with HVAC and BMS for real-time synchronization
- Log events (door openings, alerts) for validation records
Recommended: Electronic interlocks controlled via PLC with HMI touchscreen, memory logging capabilities—ideal for weighing rooms, mixing areas, and primary airlocks.
Cosmetics industry: compact design - user-friendly operation
In cosmetic plants, especially medium-scale GMP Cosmetic (ISO 22716) facilities, interlocks should:
- Be compact and easy to operate for frequently moving personnel
- Prioritize aesthetic appearance, with color schemes matching facility interiors
- Operate smoothly and quietly, minimizing industrial feel
Recommended: Modular 2-3 door electronic interlocks, flat-surface controls for easy cleaning. Consider pairing with proximity sensors for touchless operation in packaging zones.

Food industry: durability - hygiene - corrosion resistance
In food processing environments, interlocks must endure:
- High humidity, grease, and mild acidic vapors
- Frequent cleaning with chemicals or pressurized water
Therefore, food-grade interlocks must:
- Use corrosion-resistant materials (stainless steel 304/316L)
- Have high IP ratings (IP54-IP65) for water and dust resistance
- Feature rounded, seamless design to prevent bacterial buildup
Recommended: Waterproof electronic interlocks with stainless steel enclosures, magnetic waterproof switches, and DC power supply for safe use in wet areas such as raw material airlocks, food packing zones, and cold storage rooms.
See more: Buffer room and its applications in clean room
7. Frequently Asked Questions about interlocks in GMP cleanrooms
1. Is interlock required in all GMP airlocks?
Yes. According to EU-GMP and WHO-GMP standards, interlocks are mandatory in all airlocks separating two cleanliness grades or cleanrooms from uncontrolled hallways. They help:
- Prevent simultaneous door openings → avoid cross-contamination
- Maintain pressure differentials → protect cleanroom integrity
Lack of interlocks in airlocks is considered a major GMP non-conformance during inspections.
2. Should small factories use mechanical or electronic interlocks?
It depends on scale and control requirements.
- Mechanical interlocks suit small factories, low-priority zones, or budget constraints. They enforce “one-door-only” logic but lack monitoring features.
- Electronic interlocks suit expanding facilities or those planning full GMP compliance. They offer connectivity to alarms, HVAC, or BMS.
Recommendation: If budget allows, choose electronic interlocks for better flexibility and future scalability.
3. Can interlocks integrate with fire alarm systems?
Yes. Modern interlock systems include fail-safe functions that respond to fire alarms or power outages. Specifically:
- In fire emergencies, all doors automatically unlock to ensure safe evacuation
- Some systems prioritize door opening in the escape direction
This integration is mandatory under fire safety codes and commonly applied in pharmaceutical, cosmetic, and food factories.
8. Contact us for interlock consultation tailored to your GMP facility
Are you:
- Designing an airlock system for a pharmaceutical, cosmetic, or food plant?
- Undecided between mechanical, electrical, electronic, or smart interlocks?
- Seeking a solution that meets EU-GMP, WHO-GMP, or ISO 22716 standards?
VCR - a specialized cleanroom equipment provider - is ready to support your team from consultation to implementation, ensuring a safe, compliant, and audit-ready interlock system.
Contact our technical team today for industry-specific recommendations tailored to your cleanroom zones.
Hotline: 090.123.9008
Email: [email protected]
Website: https://khoaliendong.com/
Diep VCR