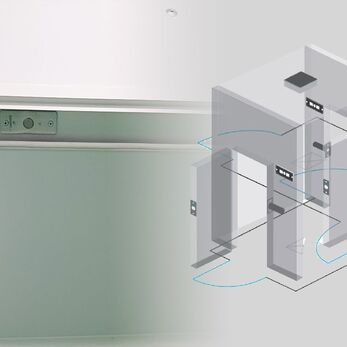
Interlock is an interlocking system that helps control the flow between clean areas, maintain differential pressure and prevent cross-contamination. This article will help you compare the advantages and disadvantages of each type to choose the most effective GMP-compliant solution.
- 1. What is an Interlock and Why is it Necessary in GMP?
- 2. Overview Comparison: Mechanical vs. Electronic Interlocks
- 3. When Should You Choose a Mechanical Interlock?
- 4. When Should You Choose an Electronic Interlock?
- 5. What Do GMP Standards Say About Interlock Systems?
- 6. Which Type to Choose? Expert Perspective
- 7. Suggested Interlock Configurations by Area (Industry-Based)
- 8. Frequently Asked Questions
- 9. Don’t Let Interlocks Be a Weak Link in Your GMP Control
1. What is an Interlock and Why is it Necessary in GMP?
An interlock is a door coordination system that ensures only one door can be opened at a time. This mechanism plays a critical role in controlling the flow of personnel and materials between cleanroom zones of different classifications, maintaining stable differential pressure, and preventing cross-contamination or reverse contamination.
Think of it this way: if a room has two entrances, the interlock ensures that when one door is open, the other must remain closed - acting like a “safety checkpoint” in your facility's traffic flow.
Interlock in GMP Standards

In industries like pharmaceuticals, cosmetics, and food manufacturing - especially in facilities adhering to Good Manufacturing Practice (GMP) - controlling movement between areas is a mandatory requirement.
According to GMP guidelines:
- Areas such as personnel airlocks, weighing rooms, gowning zones, and filling areas must not allow simultaneous access between environments of differing cleanliness levels.
- Interlocks are either required or strongly recommended as the technical solution to enforce this control.
Thus, an interlock is not merely a technical device - it is a critical control point in GMP-compliant operations and validation processes.
2. Overview Comparison: Mechanical vs. Electronic Interlocks
In a GMP cleanroom environment, choosing the right interlock depends not only on budget but also on how strict the control requirements are for each area. The table below provides a general comparison between mechanical and electronic interlocks:
|
Criteria |
Mechanical Interlock |
Electronic Interlock |
|
Operating Mechanism |
Functions via simple mechanical linkage, no electricity needed |
Uses control circuits, door position sensors, and electronic relays |
|
Flexibility |
Fixed setup, minimal configurability |
Highly configurable: number of doors, logic flow, timing, etc. |
|
Monitoring Capability |
No status indicators |
Equipped with control panels, LED indicators, alarm systems |
|
Maintenance |
Easy to maintain, no technical expertise required |
Dependent on electronic components, requires skilled maintenance |
|
Initial Cost |
Lower cost, ideal for secondary areas |
Higher upfront cost but multifunctional and cost-effective over time |
See more: Why is interlock a mandatory device in GMP airlock?
3. When Should You Choose a Mechanical Interlock?
Not every area in a facility requires the complexity of an electronic system. In many cases, a mechanical interlock is a smart, budget-conscious choice that still satisfies basic GMP requirements.
Best suited for low-risk support zones:
- Outer gowning rooms: connecting corridors to primary clean areas.
- Secondary storage or utility rooms: non-critical spaces without direct production activity.
- Technical or emergency exits: need only basic door interlock, no monitoring features.

Ideal for small-scale or budget-constrained operations:
- SMEs or new GMP-compliant facilities in the initial build-out phase.
- Where investment prioritization is necessary, mechanical interlocks offer a reliable alternative.
Key Advantages:
- No power dependency: Operates even during power outages - ideal for locations with unstable electricity.
- Simple, robust construction: No software, no programming, minimal risk of malfunction.
- Resilient in harsh environments: Performs well in humid or dusty conditions without electronic failures.
4. When Should You Choose an Electronic Interlock?
In GMP-certified manufacturing, particularly in high-contamination-risk zones, electronic interlocks are essential for achieving both regulatory compliance and automation efficiency.
Suitable for critical production areas:
- Personnel and material airlocks: buffer zones between clean levels where strict door sequencing is vital.
- Weighing rooms: where powder particles pose cross-contamination risks.
- Sampling and direct packaging rooms: require highly stable and controlled environments.
- Facilities integrating BMS/EMS systems, or those targeting digital transformation in GMP oversight.

Key Benefits of Electronic Interlocks:
Real-time Monitoring & Alarm Functions:
- Display door status (open, closed, locked)
- Sound alarms for improper operation (e.g., simultaneous door opening)
Integration with GMP Systems:
- Record operation logs (audit trails) - critical for GMP inspection and validation
- Compatible with Building Management Systems (BMS) or Environmental Monitoring Systems (EMS)
Flexible Configuration:
- Supports 2-4 doors depending on room layout
- Programmable logic tailored to each cleanroom zone
Enhanced Safety & Operational Clarity:
- LED indicators for door status
- Emergency lock release in case of incidents
See more: Latest price list of Interlock used in food factory
5. What Do GMP Standards Say About Interlock Systems?
In GMP (Good Manufacturing Practice) guidance documents, interlock systems are not merely a recommendation - they are a mandatory requirement for areas with strict sanitary control.
No specific type is mandated…
Standards such as EU‑GMP, WHO‑GMP, PIC/S do not explicitly require mechanical or electronic interlocks. What they do require is:
- No simultaneous opening of two doors that lead into a cleanroom or higher classification clean area.
- A technical mechanism must enforce that logic - whether via mechanical linkage (mechanical interlock) or electronic control (electronic interlock).
Preference for electronic solutions in high-risk zones
In technical notes and audit practice, electronic interlocks are strongly recommended in these critical areas:
- Weighing rooms for raw materials
- Personnel airlocks entering high-class clean zones
- Sampling rooms for raw materials
- Sterile packaging areas
Reasons include:
- Easier to verify the event history (who opened, when, which door opened first…) - essential for GMP audit trails.
- Less reliance on human operators - reducing errors such as opening the wrong door or forgetting to close a door.

Design considerations under GMP
- If using a mechanical interlock, you must have strict SOPs and proper training for staff to follow sequence protocols.
- If using an electronic interlock, choose models that store event data, support alarms or alerts, and facilitate validation/inspection.
See more: Why Cleanrooms Depend on Door Interlock Systems: Maintaining Airtight Integrity
6. Which Type to Choose? Expert Perspective
Choosing between mechanical and electronic interlocks should not be driven solely by cost. The decision should consider intended use, risk level, and long-term facility strategy. Here are expert suggestions from VCR:
|
Purpose / Use Case |
Recommended Device Type |
|
Only need basic prevention of simultaneous door opening |
Mechanical interlock: cost‑effective, easy to install, no electricity needed |
|
Need to fully meet GMP control requirements |
Electronic interlock: supports door status monitoring, event logging, fault alarms |
|
Smart factory orientation, automation vision |
Electronic interlock + BMS integration: connect to central control and increase monitoring |
Key factors to consider when selecting an interlock:
- The risk of cross-contamination by area
- The frequency of passage through the door
- QA / GMP inspector requirements
- Capability to integrate with central management systems (BMS/EMS)
- Investment budget and long‑term operating cost
7. Suggested Interlock Configurations by Area (Industry-Based)
Choosing the right type of interlock depends on the characteristics of each area and the manufacturing industry. Below is a table of recommended configurations by VCR’s technical team, aligned with GMP requirements:
|
GMP Area |
Recommended Interlock Type |
Technical Notes / Remarks |
|
Pharmaceutical weighing room |
Electronic interlock |
Include alarm if door sequence is violated; maintain event logs for audit |
|
Personnel airlock |
Mechanical or electronic interlock |
Use mechanical in low-control zones; use electronic when near high-grade clean areas |
|
Cosmetic filling / bottling area |
Electronic interlock |
Add door status indicator lights; alarm on incorrect operation |
|
Gowning zones ISO 8 → 7 → 5 |
3-door electronic interlock |
Alert if garments are removed or door flow is reversed (violating clean flow) |
Industry-specific design notes:
- Pharmaceuticals: highest demands on contamination control → prefer electronic interlocks with remote supervision, logging, and audit features.
- Cosmetics: though electronic interlocks are not always mandatory, they are recommended for filling/packaging areas to boost reliability under ISO 22716 standards.
- Food: for volatile or microbial-sensitive products, electronic interlocks are better in raw material and personnel airlocks.
- Electronics / semiconductors: careful control of pressure and static discharge is essential - consider interlocks combined with pressure sensors and door status monitoring.
See more: Different Categories Of Cleanroom Interlock
8. Frequently Asked Questions
- Is an electronic interlock mandatory under GMP?
No, it is not mandatory. However, it is strongly recommended in high-risk zones like weighing rooms, airlocks, and filling areas. GMP requires that you prevent simultaneous opening of two doors; whether you use mechanical or electronic solutions depends on your control needs and monitoring requirements. - Can you upgrade from mechanical to electronic interlock?
Absolutely. Many facilities adopt a retrofit approach: retain existing door hardware and install an electronic control unit to enhance oversight and data connectivity. - Are electronic interlocks prone to failure?
Electronic systems depend on components and control circuitry, but if you choose a high-quality product and install it correctly, reliability is high. Tip: select brands that offer long-term warranties, responsive technical support, and readily available spare parts. - Is regular maintenance required for electronic interlocks?
Yes. Especially in critical zones such as pharmaceutical weighing rooms and central airlocks, schedule maintenance every 3-6 months to inspect:
- Door position sensors
- Indicator lights and alarm circuits
- Event logging and data retrieval
Proper maintenance prolongs device lifespan and ensures long-term GMP compliance.
9. Don’t Let Interlocks Be a Weak Link in Your GMP Control
The interlock system is not just a piece of equipment - it is a crucial link in your contamination control chain and GMP compliance.
Do you need a flexible solution that fits your cleanroom layout?
Are you torn between mechanical and electronic interlocks for different zones?
Do you want devices that support BMS integration, automatic logging, and real-time alerts?
VCR can help.
Let our technical experts consult on interlock configurations tailored to your cleanroom layout, industry, and compliance needs.
Contact us today for free 1:1 technical consultation or review our line of electronic interlock solutions at:
Hotline: 090.123.9008
Email: [email protected]
Website: https://khoaliendong.com/
Diep VCR