Cleanroom door interlock is a safe solution that helps control the logical opening/closing of doors, prevents cross-contamination and maintains stable pressure. Choosing a reputable supplier of GMP-standard Cleanroom Door Interlock will ensure the system operates effectively, meeting GMP/ISO requirements.
- 1. What is a Cleanroom Interlock System? What are the types?
- 2. Industries That Require GMP-Compliant Interlock Systems
- 3. Key Criteria When Choosing a Trusted Interlock Supplier
- 4. Why Choose VCR - Cleanroom Interlock System Specialist
- 5. Common Interlock Systems Provided by VCR
- 6. Case Study: Interlock System for EU-GMP Pharmaceutical Facility
- 7. Frequently Asked Questions About Cleanroom Interlock Systems
- 8. Need Consultation for a GMP-Compliant Interlock System?
1. What is a Cleanroom Interlock System? What are the types?
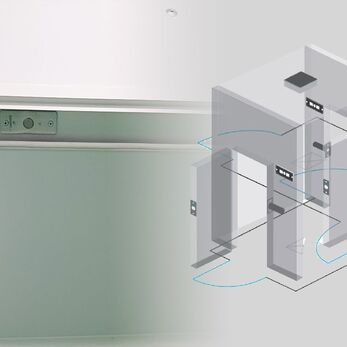
A Cleanroom Interlock System is a control mechanism that regulates the opening and closing of multiple doors based on safety logic, ensuring that only one door can be opened at a time. This helps prevent cross-contamination, pressure loss, and the intrusion of airborne particles between different zones in the cleanroom.
These systems are typically installed in transitional areas such as airlocks, gowning rooms, weighing rooms, and clean corridors to maintain positive or negative pressure and restrict contaminated air from moving across clean zones.

Common types of interlock systems:
|
Type of Interlock |
Operating Mechanism |
Key Benefits |
Suitable Applications |
|
Mechanical |
Mechanical latch between two doors, no electricity required |
Simple, low-cost |
Storage rooms, low-critical zones |
|
Semi-automatic |
Sensor + electric bolt, manual push-button |
Flexible, easy installation |
Gowning rooms, small airlocks |
|
Electronic |
Programmable logic controller, smart sensors, control panel |
High precision, scalable, system integration |
Weighing rooms, GMP-grade airlocks |
Modern interlock systems can also integrate with alarm systems (light/sound), building management systems (BMS), or access control to enhance automation and meet GMP and ISO 14644 standards in pharmaceutical, food, cosmetic, and electronics industries.
2. Industries That Require GMP-Compliant Interlock Systems
In industries where strict cleanroom control is mandatory, interlock systems are not optional — they are required to ensure unidirectional movement, pressure control, and cross-contamination prevention.
Here are four key sectors that must implement GMP-compliant interlock systems:
Pharmaceutical Industry - Pressure Control & Cross-contamination Prevention
According to EU-GMP and WHO-GMP standards, areas such as airlocks, weighing rooms, and gowning rooms must be equipped with interlock systems to maintain proper pressure differentials (typically positive pressure between clean zones).
These systems prevent airborne contaminants, dust, or APIs from spreading between rooms—especially important in processes involving high-potency compounds or antibiotics.
Typical applications: Airlocks between corridors and production rooms, 3-door interlocks in gowning areas (Grade D → C → B), interlocked access to high-risk weighing rooms.
Food Industry - Contamination Prevention
Food manufacturing facilities face a high risk of microbial contamination, especially in areas such as preparation, filling, and packaging.
Interlock systems help limit the number of door openings, maintain clean airflow, and block contaminants from personnel, equipment, and materials.
They are particularly valuable in factories certified to HACCP or ISO 22000 standards.

Cosmetic Industry - Ensuring Unidirectional Flow
Cosmetic production requires strict control of personnel and material flow to avoid cross-flow and reverse contamination.
Installing interlocks in gowning rooms, filling stations, and packaging zones is essential for compliance with GMP or ISO 22716 standards.
These systems are often integrated with UV lights, pass boxes, and safety sensors.
Electronics Industry - Dust and Static Control
Electronics manufacturing demands ultra-clean environments for processes such as PCB, chip, and semiconductor production.
Interlocks help maintain stable pressure, prevent dust ingress, and control personnel entry/exit to reduce static risk.
They are commonly integrated with FFUs, ESD systems, ionizers, and pass boxes.
See more: Different Categories Of Cleanroom Interlock
3. Key Criteria When Choosing a Trusted Interlock Supplier
Installing a cleanroom interlock system is not just purchasing a device — it involves selecting a technical solution tailored to GMP-compliant processes. Below are key criteria when evaluating a supplier:
In-depth Knowledge of GMP & ISO 14644
- The supplier must understand cleanroom design requirements, especially for airlocks, weighing rooms, and gowning areas.
- Capable of advising on door logic sequencing based on cleanroom classification, pressure differentials, and workflow.
- Familiarity with standards such as EU-GMP, WHO-GMP, and ISO 14644-4 is essential.
End-to-End Solutions: Consultation - Installation - Maintenance
A reliable supplier should not only provide equipment but also:
- Design interlock logic diagrams
- Perform installation in line with construction milestones
- Deliver operation manuals and documentation
- Offer regular maintenance and technical support
This ensures long-term system stability and minimizes risks that could disrupt production.

Full Documentation: CO, CQ, Technical Manuals
Every product must include:
- CO (Certificate of Origin) - confirms origin
- CQ (Certificate of Quality) - confirms product quality
- User manuals for installation and operation
- Wiring diagrams and interlock logic charts
These documents are often mandatory during GMP audits.
Compatibility with Cleanroom Systems
The interlock system must be compatible with:
- Panel doors, glass/aluminum doors, stainless steel doors
- Automatic, sliding, or manual swing doors
- Access control systems
- Auxiliary cleanroom equipment like pass boxes, air showers, and FFUs
Choose a supplier with a broad product portfolio and modular solutions that are easy to scale or upgrade.
See more: Buffer room and its applications in clean room
4. Why Choose VCR - Cleanroom Interlock System Specialist
Choosing a supplier for cleanroom interlock systems should not be based on price alone—it requires a comprehensive evaluation of their technical expertise, solution quality, and industry knowledge. VCR is one of the few providers in Vietnam capable of delivering internationally compliant cleanroom interlock solutions, thanks to the following key advantages:
Over 10 years of experience in cleanroom engineering
VCR has partnered with hundreds of GMP-certified projects over the past decade.
Our engineering team is well-versed in EU-GMP, ISO 14644, and HACCP standards and is capable of providing consultation from layout design to system validation and audit support.
Fully integrated cleanroom equipment ecosystem
VCR supplies a complete suite of compatible cleanroom systems, including:
- Interlock systems, panel doors, interlock controllers
- Pressure differential alarms, indicator lights, pass boxes, FFUs
All devices are designed to work seamlessly together, minimizing compatibility issues that often occur when components are sourced from multiple vendors.
Industry-specific consulting
Each industry has its own technical requirements. VCR offers tailored solutions for specific sectors:
- Pharmaceuticals: Cross-contamination control, weighing rooms, airlocks
- Food: Personnel flow management, insect prevention, hygiene compliance
- Cosmetics: Filling areas, packaging zones, gowning rooms
- Electronics: ESD control, ISO 5-6 critical areas
See more: Kawaoka A2 Series - Cleanroom Interlock System
5. Common Interlock Systems Provided by VCR
VCR offers a wide range of cleanroom interlock systems, from basic models to advanced solutions, meeting the needs of both small-scale facilities and large GMP-compliant factories. The three most common types are:
|
Interlock Type |
Technical Features |
Suitable Applications |
|
Mechanical Interlock |
- No power required - Mechanical latch between two doors - Simple and durable operation |
- Raw material storage - Auxiliary zones without strict cleanroom requirements |
|
Semi-Automatic Interlock |
- Sensor-based with electric bolt - Push-button door activation - Integrated alarm lighting |
- Weighing rooms - Small airlocks in GMP plants |
|
Smart Electronic Interlock |
- Programmable logic controller - Real-time door status monitoring - Alarm when door logic is violated |
- EU-GMP pharmaceutical facilities - High-containment and pressure-sensitive zones |
Advantages of choosing VCR interlock systems:
- Wide selection based on control level and investment budget
- Seamless integration with panel doors, automatic doors, pass boxes, access control systems
- Customized door logic consulting based on your facility layout
- All products come with CO, CQ, wiring diagrams, and operation manuals, ready for GMP audit anytime
6. Case Study: Interlock System for EU-GMP Pharmaceutical Facility
A pharmaceutical factory operating under EU-GMP standards requires extremely strict environmental control, especially in transitional zones such as airlocks, gowning rooms, and weighing rooms. Below is an example of how VCR deployed a fully integrated interlock system for a real-world project:
Interlock installation locations:

- Three-stage gowning room (Grade D → C → B):
Each stage is equipped with 2-3 door interlocks, ensuring only one door opens at a time to prevent airflow reversal.
Integrated with door status lights and audible alarms for logic violations. - Personnel and material airlocks:
Installed between clean corridors and production areas.
Includes pass boxes with dedicated interlock systems.
Uses magnetic sensors and electric bolts configured to preset logic. - High-risk weighing room:
Equipped with smart electronic interlocks, programmed to open/lock based on pressure differential.
Includes push-button entry with user authentication.
Doors automatically lock if the pressure threshold is not met.
Interlock logic diagram: Optimizing flow and pressure control
- Magnetic sensors: Monitor door open/close status
- Central logic controller: Programs door opening sequence, time delay, and error alerts
- Internal push-buttons: Installed in each area for safe and convenient access
- Red/green lights with alarms: Warn operators if logic is breached
Integration with other cleanroom systems:
- FFU (Fan Filter Units): Synchronized with door state to maintain clean airflow
- Pressure sensors and differential monitors: Lock doors if pressure is not within threshold
- Access control systems: Integrated with RFID readers to assign entry rights by area or shift
Results:
- Optimized personnel and material flow in compliance with GMP
- Significantly reduced cross-contamination and pressure imbalance risks
- Easy to operate and maintain, fully compliant with international audit requirements
See more: Latest price list of Interlock used in Cosmetic factory
7. Frequently Asked Questions About Cleanroom Interlock Systems
Is it mandatory to use interlock systems in pharmaceutical cleanrooms?
Yes. According to GMP standards, interlock systems are required in areas such as airlocks, weighing rooms, and gowning rooms to:
- Prevent multiple doors from opening simultaneously, avoiding microbiological cross-contamination
- Maintain stable pressure differentials between cleanroom classifications
- Reduce reverse airflow that can compromise production quality
How many doors can a single interlock system control?
It depends on the type of controller. A standard interlock system can:
- Control 2 to 4 doors
- Expand to 6 or more doors using a central controller with multiple input/output ports
- Be programmed with logic for door priority, delay timing, or sensor-based conditions
Can interlock systems integrate with pressure differential monitoring?
Yes. Modern electronic interlock systems can:
- Read input from pressure sensors
- Prevent doors from opening if the pressure is out of specification
- Automatically open doors or trigger light/sound alarms when deviations occur
- Integrate with systems such as FFUs, alarms, and access control
8. Need Consultation for a GMP-Compliant Interlock System?
VCR is a leading provider of cleanroom interlock systems, with over 10 years of hands-on experience delivering solutions for pharmaceutical, food, cosmetics, and electronics factories.
- Technical consulting tailored by industry and facility layout
- Full-package delivery: equipment, installation, documentation (CO/CQ)
- System-wide integration with panel doors, pass boxes, FFUs, pressure monitoring, and more
Contact us today to receive a free interlock logic diagram demo:
Hotline: 090.123.9008
Email: [email protected]
Website: https://khoaliendong.com/
Diep VCR